Accurately assessing patients’ risk for CHD events remains a significant challenge in primary prevention.
In a large cohort of patients hospitalized with coronary artery disease, nearly 50% had admission LDL levels <100 mg/dL.2
The PLAC® Activity Test is a strong and independent risk factor
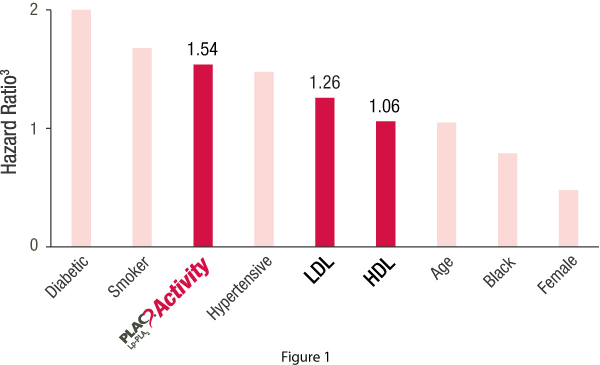
The FDA cleared PLAC Activity Test showed a strong association with CHD events* as compared to traditional risk factors even after adjusting for age, gender, race, diabetes and smoking status. Only complex clinical conditions such as diabetes and smoking status demonstrated greater hazard ratios for CHD events.
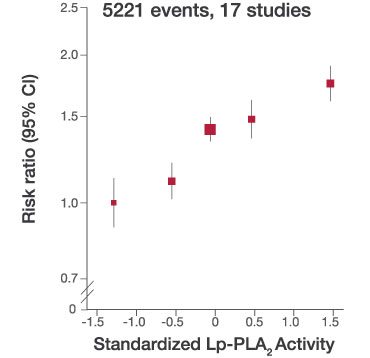
Effectiveness of the PLAC Activity Test has been demonstrated across multiple clinical trials and patient populations.
- The greater the Lp-PLA2 activity, the greater the risk for fatal and nonfatal CHD events (Figure 1)
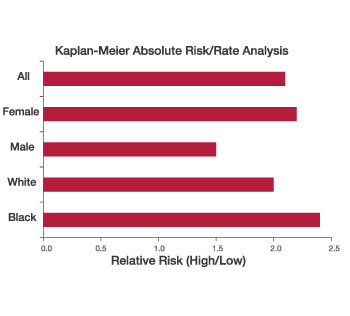
- A PLAC Activity Test result above 225 nmol/min/mL identifies patients at increased risk for CHD events across patient type and population (Figure 2)
- Absolute risk of CHD events is 2.1 times greater with a positive PLAC Test (Figure 2)
 |
The PLAC® Activity Test incorporates a validated, easy-to-use cut point of 225 nmol/min/mL. |
HEALTHCARE PROFESSIONALS
he PLAC Activity Test provides physicians with additional prognostic information previously unavailable with traditional risk factors.
LABORATORY PROFESSIONALS
The PLAC Activity Test offers enhanced cardiovascular risk assessment from your laboratory.
*Myocardial Infarction, cardiac revascularization, cardiac death.
References: 1. US Preventive Services Task Force. Final recommendation statement: lipid disorders in adults (cholesterol, dyslipidemia): screening, June 2008. http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/lipid-disorders-in-adults-cholesterol-dyslipidemia-screening#. Published December 2014. Accessed April 29, 2015. 2. Sachdeva A, Cannon CP, Deedwania PC, et al. Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get With The Guidelines. Am Heart J. 2009;157(1):111-117.e2. 3. 3rd Annual American Society for Preventive Cardiology Cardiovascular Disease Preventive Conference, 2015. Symposium of REGARDS Lp-PLA2 Substudy. 4. Thompson A, Gao P, Orfei L, et al; Lp-PLA2 Studies Collaboration. Lipoprotein-associated phospholipase A2 and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375(9725):1536-1544. 5. PLAC® Test for Lp-PLA2 Activity [package insert]. South San Francisco, CA: Diadexus, Inc; 2015.